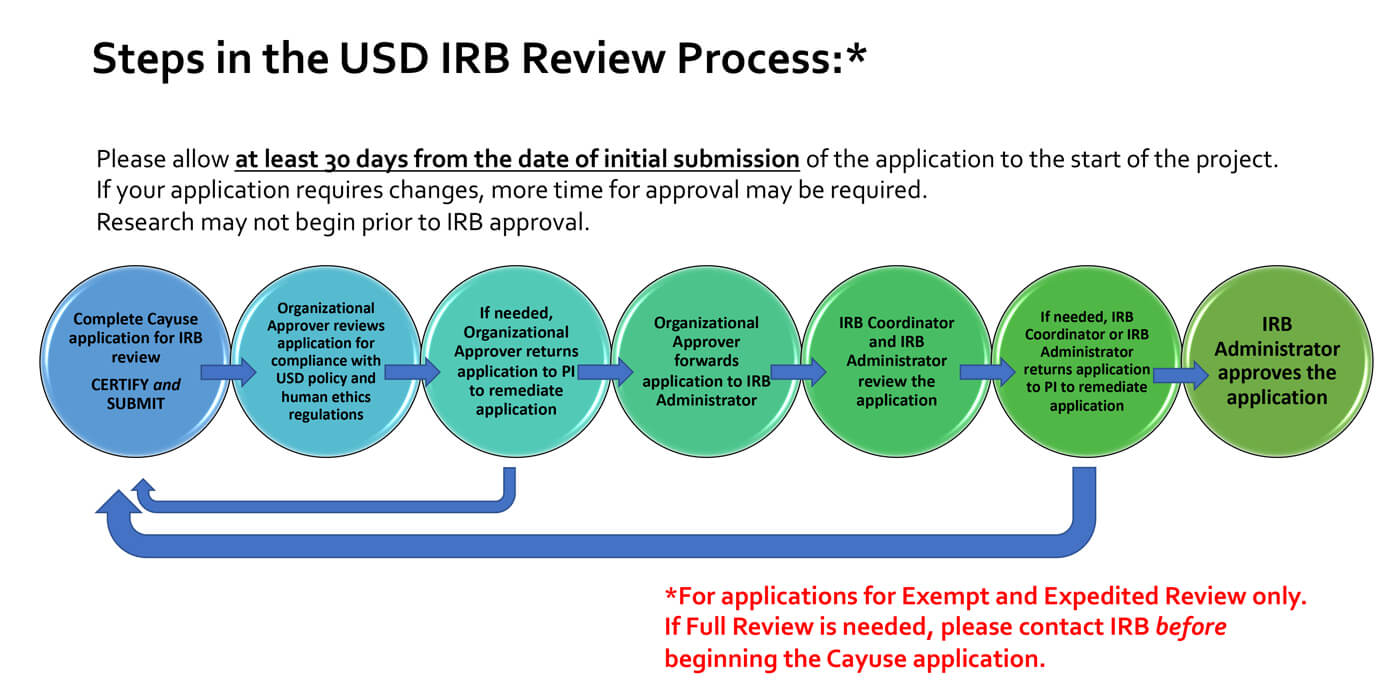
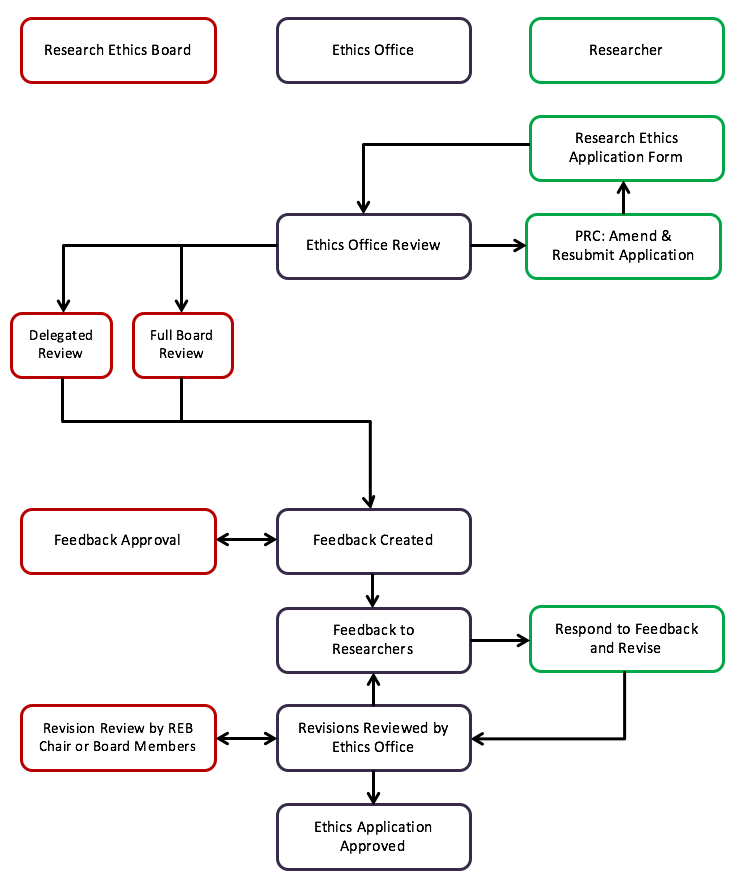
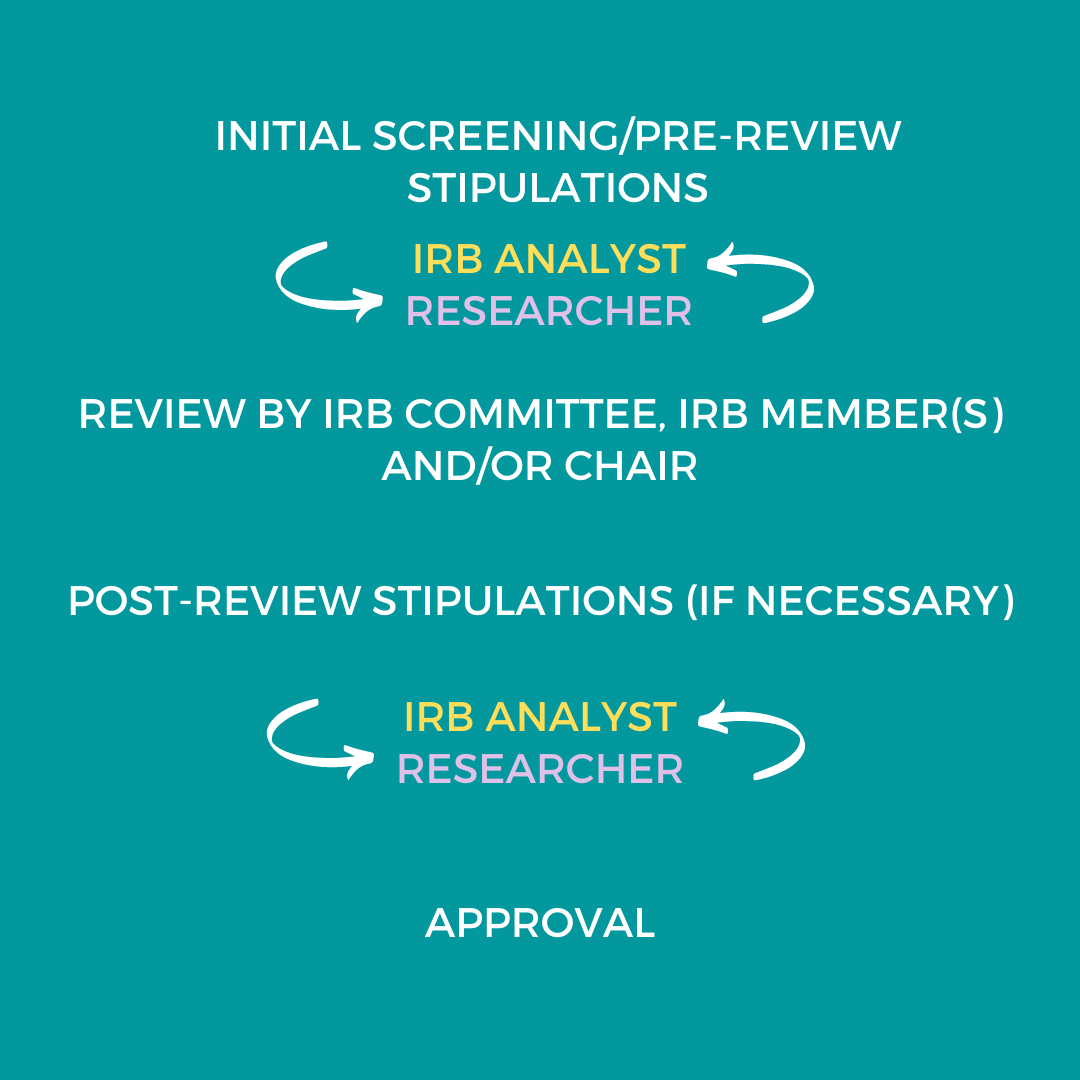
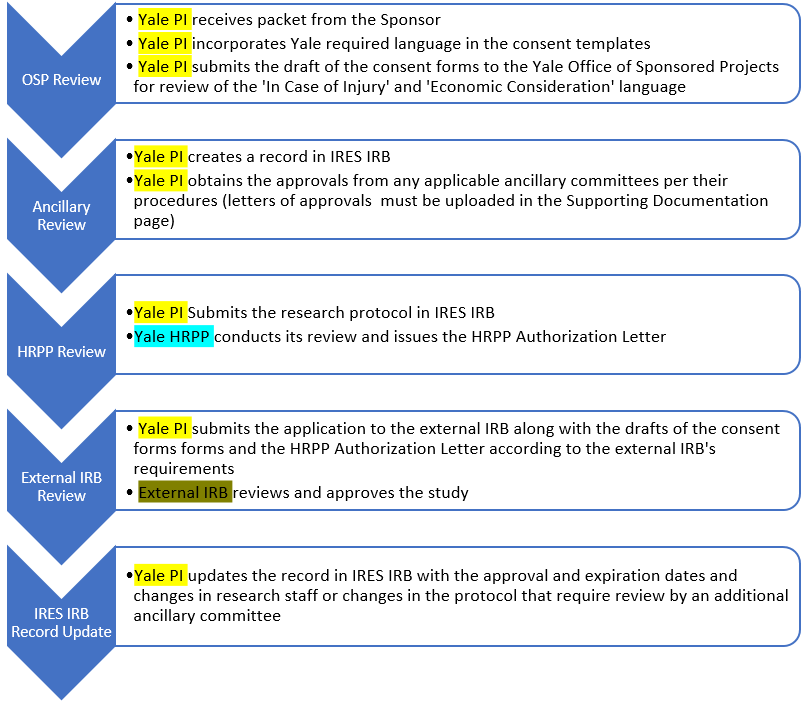
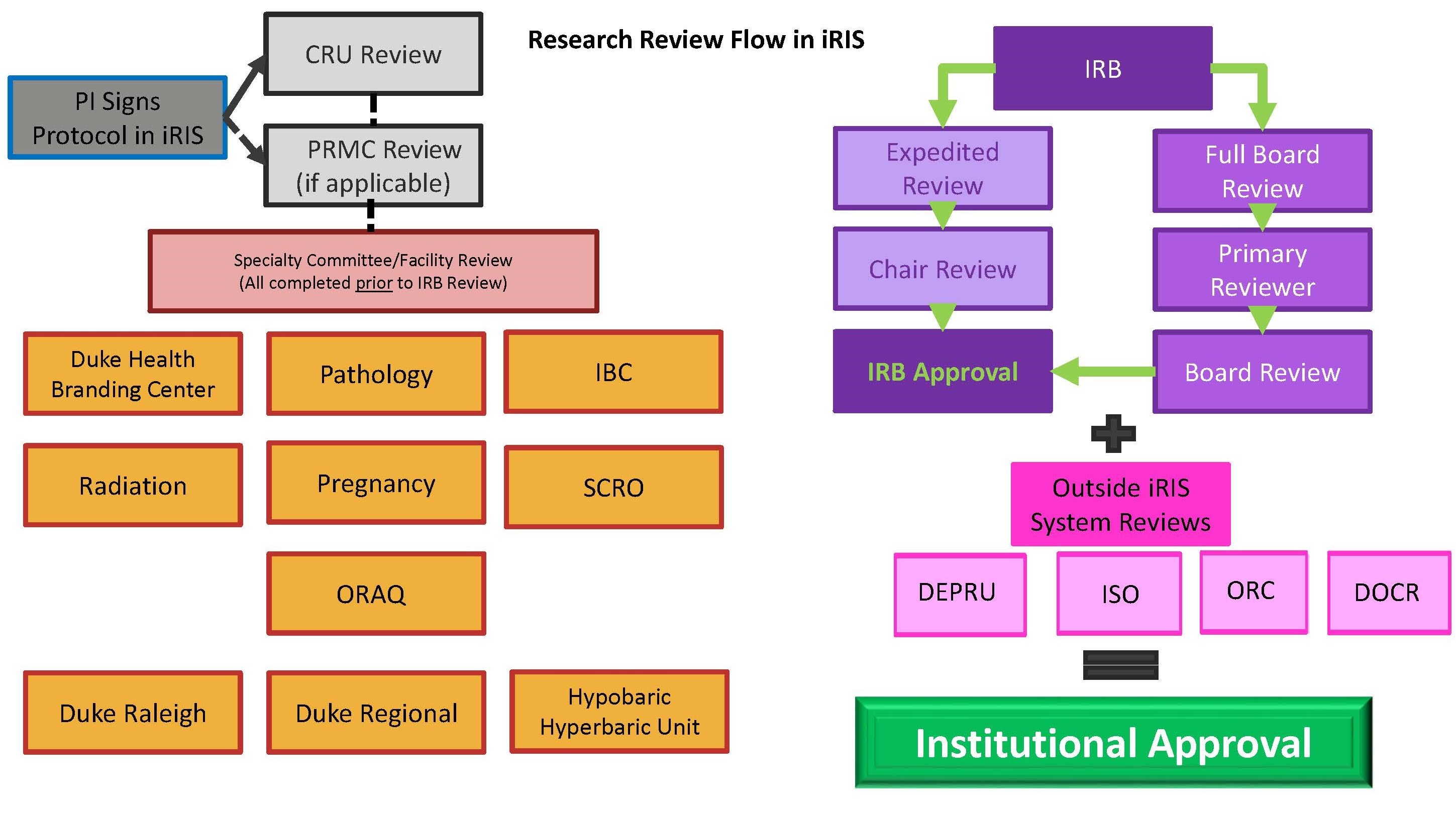
RESEARCH ETHICS BOARD STANDARD OPERATING PROCEDURES SOP Number 404 Ongoing REB Review Activities Date of Issue 2022 07 13 Purp
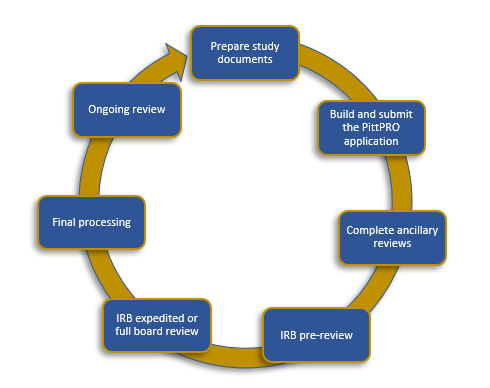
RESEARCH ETHICS BOARD STANDARD OPERATING PROCEDURES SOP Number 403 Initial Review - Criteria for REB Approval Date of Issue Dat
Doctoral Dissertation Research and the IRB | 2021 | IRB Blog | Institutional Review Board | Teachers College, Columbia University

Requirements for Institutional Review Board (IRB) Review and HIPAA Waiver Documentation for RIF DUA Request Submissions | ResDAC

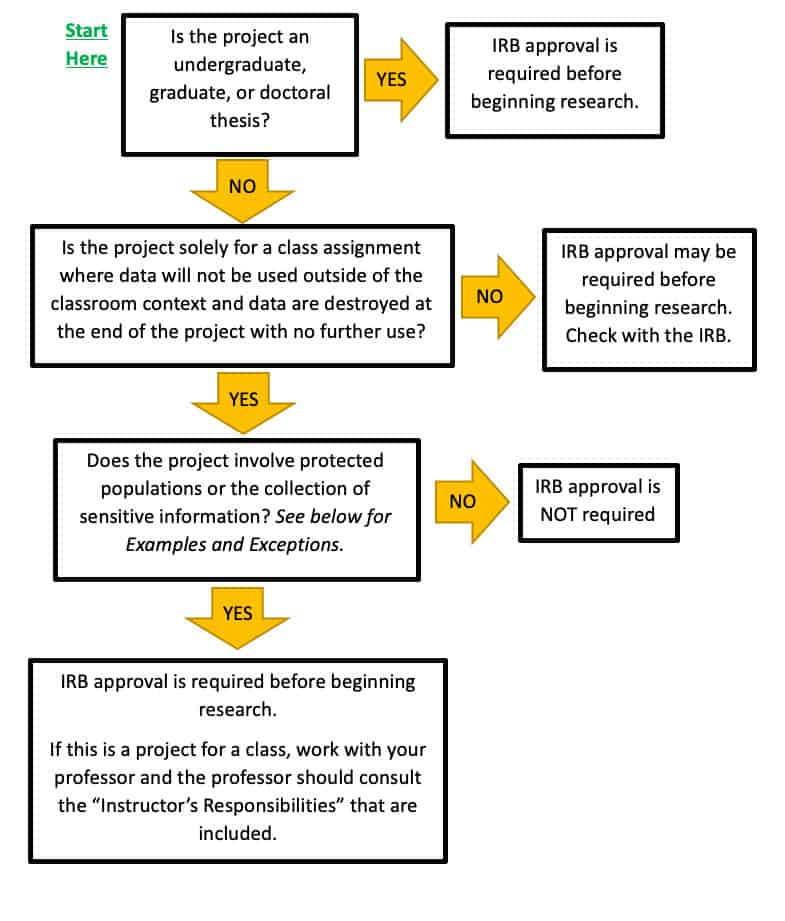
Do I really need IRB approval for my SoTL project? – Center for Innovative Teaching and Learning @IUB
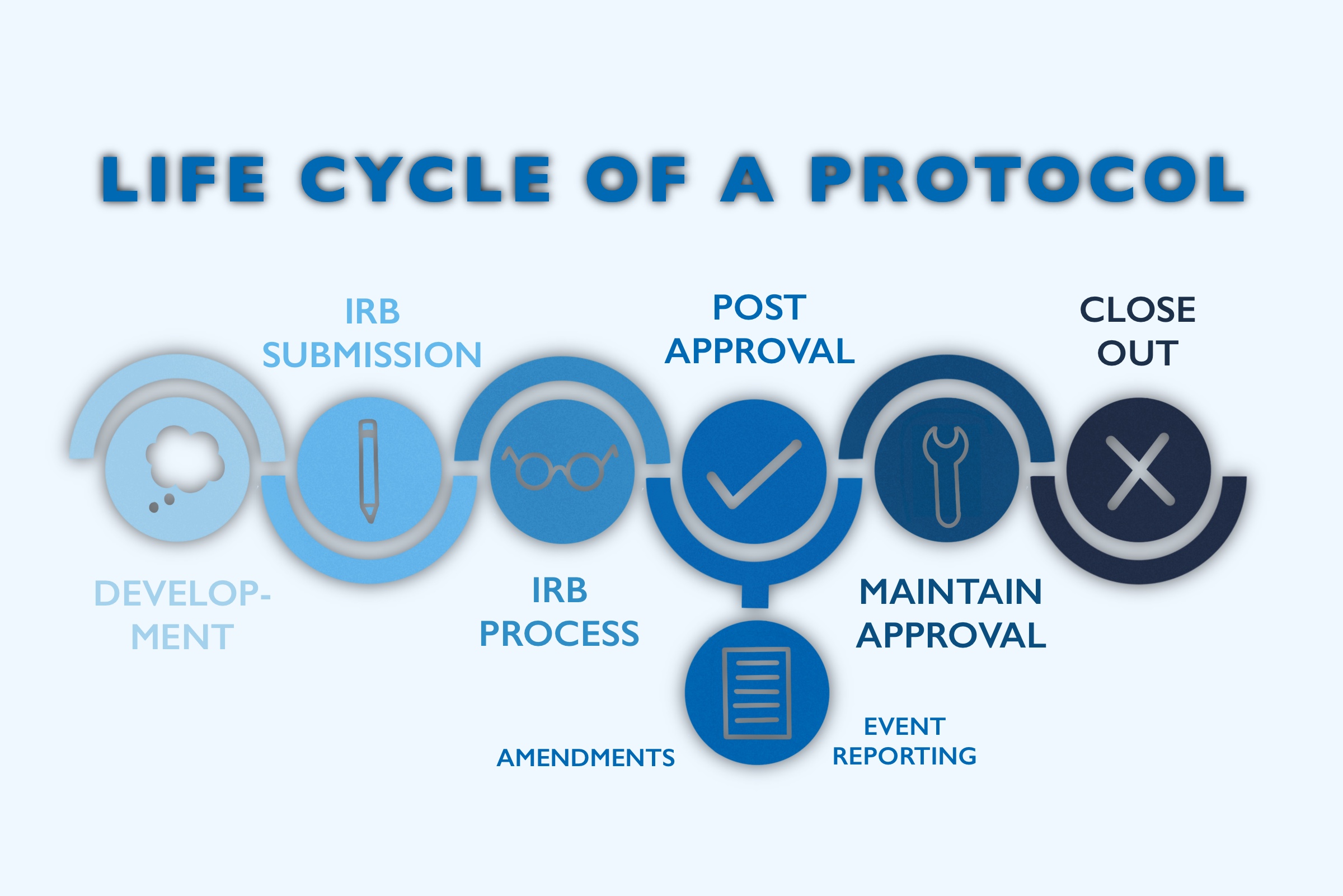
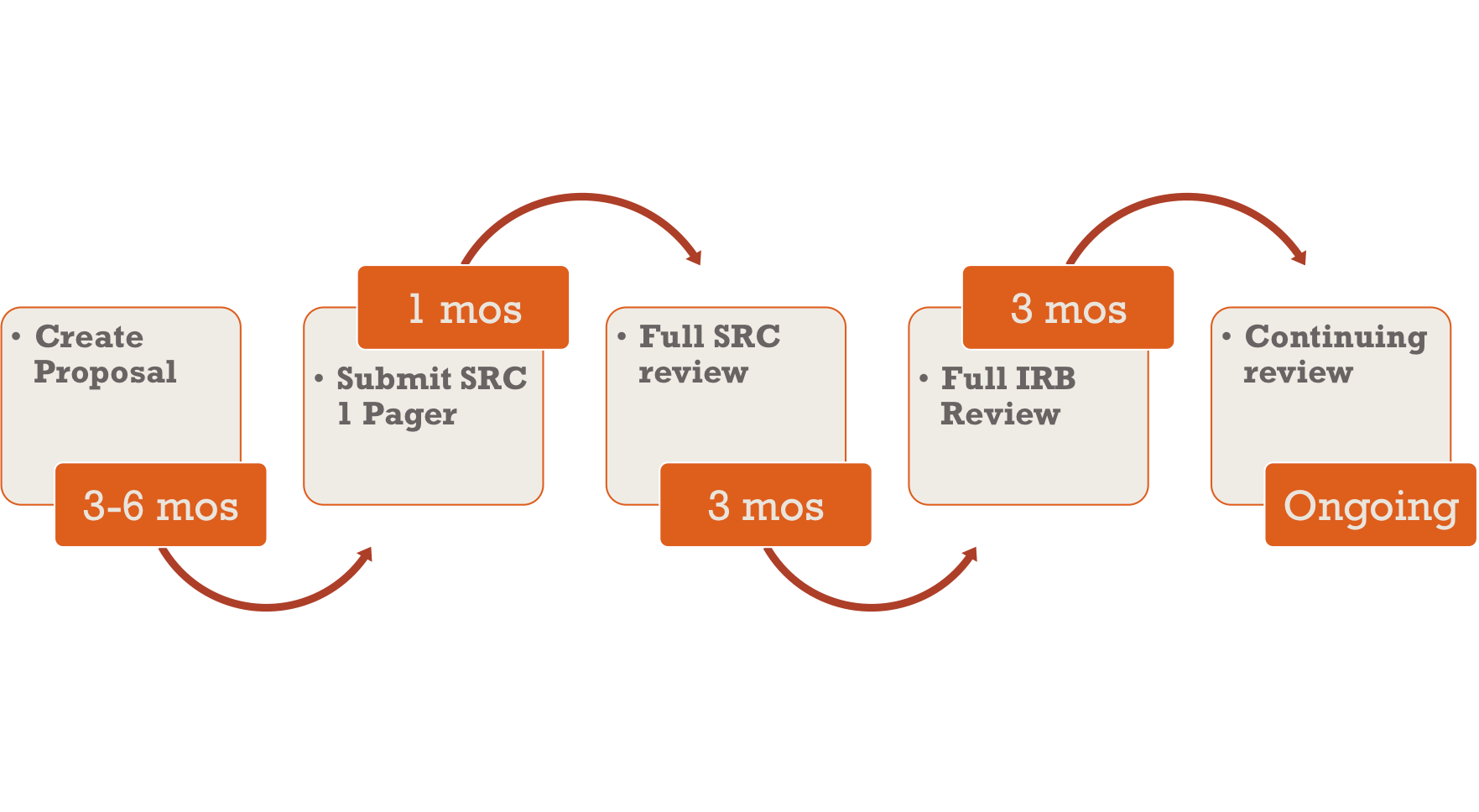
How do Institutional Review Boards (IRB) and Ethics Committees (EC) impact clinical trials? - Clincierge